Correction: Pfizer’s vaccine is not FDA approved; it’s authorized.
There is a consensus that the Food and Drug Administration (FDA) is rushing the decision to “approve” Pfizer’s Covid-19 vaccine. Before rushing to that conclusion, let’s understand the FDA’s vaccine approval process at a glance.
Firstly, it is important to note that even though the Pfizer vaccine was 95% effective at preventing cases of COVID-19 in both Latinos and non-Latinos, 100% effective in Black people, 94% effective in people who were at least 56 years old, 95% effective in those who had at least one medical condition, and 96% effective for people who were obese… It was still not enough to receive “Approval” from the FDA.
Instead, it was granted an emergency use “Authorization” (EUA), which is still a very high standard to meet. An EUA can be granted to anything used to “diagnose, treat, or prevent serious or life-threatening diseases or conditions”. Another requirement for issuing authorization is “there are no adequate, approved, and available alternatives” to the product being authorized. This EUA process is the agency’s alternative evaluation process designed to vet things more quickly and is used during public health crisis (SARS, COV19, etc.).
There is one caveat, an EUA can last only as long as a public health emergency is in effect.
If the FDA finds that the benefits of the vaccine are no longer outweighing the harm, EUA authorizations can be revoked. In that case, vaccine developers will need to continue the regular vaccine development process. Below is a summary of the FDA’s vaccine “Approval“ process:
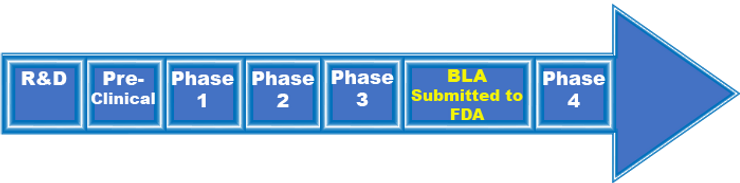
R&D: 2-4 years
Pre-Clinical: 1-2 years
Investigational New Drug (IND) Application: 30 days
Vaccine Trials
- Phase 1 Vaccine Trial: 20-80 subjects – Goal is to assess safety and immune system response in candidates
- Phase 2** Vaccine Trial: Several hundred subjects- Goal is to study the candidate vaccine’s safety, immunogenicity, proposed doses, schedule of immunizations, and method of delivery.
- Phase 3 Vaccine Trial: Must have minimum of 3000 subjects- Goal is to assess vaccine safety and efficacy in a large group of people. At least half of subjects should be tracked for at least two months after receiving their final dose
Biologics License Application (BLA) submission to FDA: FDA will inspect the factory where the vaccine will be made and approve the labeling of the vaccine.
Phase 4- Optional studies that drug companies may conduct after a vaccine is released. The manufacturer may continue to test the vaccine for safety, efficacy, and other potential uses.
To learn more about the FDA’s emergency use authorization please click on the following link: